Good policy! In 2021, many governments in my country will issue documents to support the industrial development of stem cell therapy, immune cell therapy, gene therapy and gene editing!
2021-12-22
In 2021, many local governments in my country issued documents to strongly support the industrial development of stem cell therapy, immune cell therapy, gene therapy and gene editing!
Beijing
1. On November 29, 2021, the leading group of the Zhongguancun Demonstration Zone issued the "Development and Construction Plan for the Zhongguancun National Independent Innovation Demonstration Zone during the "14th Five-Year Plan" period (hereinafter referred to as the "Plan").
The "Planning" points out: strengthen the layout of key technology fields and support disruptive technological innovation. Support the development of life science and technology research such as brain science and brain-like research, gene editing, stem cell and regenerative medicine, single-cell multi-omics, synthetic biotechnology, and biological breeding.
Strengthen the transfer and transformation services of achievements in colleges and universities, and in the field of biomedicine, give play to the role of technical platforms such as medical device engineering platforms, biological sample banks, and biocompatibility evaluation platforms, and build cell and gene therapy, biological drugs, and recombinant protein drugs. Production platform, AI innovative drug incubation platform, etc. Carry out the identification of pilot test bases, and provide special financial support for qualified pilot test bases.
Prospective layout of future industries, keen grasp of future industry development trends, and enhance sensitivity and judgment on new tracks. Support the free exploration and research of future industries related to the overall economic and social development and major leading roles, and forward-looking deployment of a number of strategic and reserve technology research and development projects. Accelerate the development of future life and health industries such as gene editing, synthetic biology, and biomanufacturing.
2. On November 24, 2021, the Beijing Municipal Committee of the Communist Party of China and the Beijing Municipal People's Government issued the "Beijing International Science and Technology Innovation Center Construction Plan during the 14th Five-Year Plan" period.
The plan clarifies the development goals and vision: by 2025, the Beijing International Science and Technology Innovation Center will be basically formed, and it will be built into the world's major scientific center and innovation highland. By 2035, Beijing International Science and Technology Innovation Center will lead the world in innovation, competitiveness and radiation.
Promote the leadership of cutting-edge technologies in key areas, and support the development of cutting-edge biotechnology research and development. In the fields of nucleic acid and quality detection, in vivo detection of cell function and pathological state, new target in vivo intervention technology, new antibody technology, gene editing, new cell therapy, stem cell and regenerative medicine, etc. Create original discoveries and establish precise diagnosis and breakthrough treatment methods for major epidemics, difficult and rare diseases.
Accelerate the cultivation of new kinetic energy of high-precision and advanced industries, and continue to strengthen the research and development of original innovative drugs with new mechanisms, new targets, and new structures such as new antibody drugs, small molecule drugs, cell and gene therapy; accelerate the development of drugs for major diseases, clinical Research and development of high-end preparations suitable for shortage of drugs and special populations, establish a new drug research and development incubation and acceleration platform, focus on supporting the discovery of new targets based on clinical cohorts, the research and development of original innovative drugs, improve the efficiency and success rate of drug research and development, support serum-free cell culture medium, Development of key raw materials and process equipment such as commercial cell lines, disposable biological reaction bags, etc. In the direction of vaccines, accelerate the development of new vaccine technologies such as messenger RNA (mRNA), and promote technological innovation and industrial system construction such as protein vaccines, vector vaccines, multivalent combined vaccines, and new vaccine adjuvants.
Improve the demonstration area of innovative industrial clusters. Build a biological drug R&D and production platform, a high-end customized R&D and production (CDMO) platform, a large-scale vaccine production base, a cell and gene therapy R&D pilot base, an mRNA vaccine technology platform and a production base, etc., to enhance the innovative support role of the medical and health platform.
3. On August 23, 2021, the Beijing Municipal People's Government issued a notice on printing and distributing the "Beijing's "14th Five-Year Plan" Period High-Precision Industry Development Plan.
The article points out: focus on three major directions: innovative drugs, new devices, and new health services, build leading advantages in new vaccines, next-generation antibody drugs, cell and gene therapy, and domestic high-end medical equipment, and promote the parallel development of pharmaceutical manufacturing and health services.
Build a gene editing platform to accelerate the development of mesenchymal stem cells, CAR-T (chimeric antigen receptor T cell therapy), oncolytic virus products, and non-viral vector gene therapy products; promote the industrialization of new vaccine varieties and production bases, macromolecular antibodies Construction of major projects such as drug production bases and macromolecular biopharmaceutical CDMO platforms.
4. On July 8, 2021, the General Office of the Beijing Municipal People's Government issued the "Beijing Action Plan for Accelerating Collaborative Innovation in Medicine and Health (2021-2023)".
The article points out that one of the key tasks of the program is to promote breakthroughs in biotechnology innovation. Support the research on cutting-edge key technologies in the field of life sciences, and produce important technological breakthroughs and international leading original discoveries in basic core technology fields such as nucleic acid and protein detection, gene editing, new cell therapy, stem cells and regenerative medicine, and promote difficult and rare Accurate diagnosis and breakthrough treatment of disease.
Continue to promote the construction of third-party professional technical service platforms such as cell and gene therapy, high-end pharmaceutical preparations, and medical device testing and verification, and promote the construction of a national animal model technology innovation center. Support and introduce international professional institutions and teams, build differentiated and characteristic professional incubators in concentrated areas of higher education, scientific research institutes, and medical institutions, and vigorously introduce internationally leading professional accelerators to land in Beijing, and cooperate with third-party research and development and cooperation. The production service platform forms a linkage and a coordinated layout.
One of the guarantee mechanisms is to strive for policy support, strengthen the connection with relevant policies of the administrative examination and approval service innovation pilots carried out by the state ministries and commissions, and actively strive for the pilot projects of human genetic resources service stations, medical device service stations, and the supervision of innovative products such as stem cells and artificial intelligence medical devices. Policy support for innovation pilot projects.
Shanghai
1. On December 6, 2021, the official website of the Shanghai Municipal People's Congress released the "Regulations on Promoting the Construction of Zhangjiang Biomedical Industry Innovation Highland in Shanghai Pudong New Area (Draft)" (draft for comments).
Article 7 (Development and Application of Human Cell and Gene Technology) states: The Municipal People's Government and the People's Government of Pudong New Area shall incorporate the promotion of the development of human cells and gene industry into the coordination and promotion mechanism for the development of the biopharmaceutical industry, and under the premise of controllable risks, support qualified The diversified investment subjects of human cells and gene technology research and development and industrialization process. The municipal and Pudong New Area science and technology, health and other departments should strengthen the supervision of the development and application of human cells and gene technologies carried out by biomedical enterprises in Pudong New Area, and strengthen risk management and control.
2. At the beginning of October 2021, the Shanghai Municipal Government issued the "14th Five-Year Plan for Shanghai to Build a Science and Technology Innovation Center with Global Influence" to accelerate the construction of a science and technology innovation center with global influence.
The article points out the key directions of biomedicine, and the key directions: in the field of innovative drug and vaccine research and development, breakthroughs in cell therapy, gene therapy, drug target discovery and confirmation, new antibody drug research and development, carbohydrate drug research and development, targeted preparations, nucleic acid interference drug research and development, etc. Key technologies to promote applications in regenerative medicine, major chronic disease treatment, tumor immunotherapy, infectious disease prevention and treatment, etc. Build major disease models that are close to clinical characteristics, accelerate the discovery of original new drugs based on novel and common biomarkers, and the research and application of new drug and vaccine design technologies, and promote new technologies, new materials, and new formulations in the development and production of new drugs and vaccines Applications. Research on key core technologies in the field of biomedicine, cell therapy and gene therapy. Establish a key technology system for cell therapy and gene therapy from laboratory to clinical stage, covering core technologies such as vector research and development, production process, quality control, and clinical transformation, to meet the growing domestic and international markets. Cell and gene therapy industry To meet the demand for chemical treatment, promote the technological innovation and industrialization of cell therapy and gene therapy products.
3. On July 21, 2021, the General Office of the Shanghai Municipal People's Government officially released the "14th Five-Year Plan for the Development of Strategic Emerging Industries and Leading Industries in Shanghai".
The plan proposes that during the "14th Five-Year Plan" period, the average annual growth rate of the biopharmaceutical industry will reach about 8%, accelerate the cultivation and growth of a number of local innovative enterprises, and continue to introduce a number of leading enterprises. By 2025, realize the independent control of a number of key core technologies such as biotechnology drugs and the preparation of new biomedical engineering products, accelerate the industrialization of innovation achievements, and basically build an innovation highland for the biomedical industry with international influence. In terms of biomedicine, focus on development: 1. Innovative drugs and high-end preparations. Focusing on new targets, new sites, new mechanisms, and new molecular entities, it focuses on the development of high-end biological products such as antibody drugs, new vaccines, gene therapy, and cell therapy, targeted chemical drugs and new preparations, and modern Chinese medicine. 2. High-end medical equipment. Focus on the development of high-end imaging equipment, high-end implantation and interventional devices and consumables, surgical treatment and life support equipment, high-end rehabilitation aids, in vitro diagnostic instruments and reagents, and biomedical materials. 3. Biotechnology services. Promote pre-clinical and clinical contract research and development (CRO), contract research and production (CMO/CDMO) and other services, and promote the integration and application of technologies such as artificial intelligence-assisted drug development and digital medical solutions. Facing the future leading industries, promote the development of gene editing, assembly, recombination and other technologies, build cell factories that can produce drugs, chemicals, natural products, and bioenergy, and promote the industrial application of synthetic biology technology. Deepen the research and development of somatic cell reprogramming, artificial tissue and organ construction, etc., and promote the clinical application of stem cell repair pathological damage, tissue and organ regeneration and other cell technologies.
4. On July 6, 2021, the General Office of the Shanghai Municipal People's Government issued a notice on the issuance of the "14th Five-Year Plan for the Development of Advanced Manufacturing in Shanghai".
The article points out that it will focus on cutting-edge biological fields such as brain science, gene editing, synthetic biology, cell therapy, stem cells and regenerative medicine, carry out major scientific and technological research, and promote equipment such as key raw materials, high-end raw materials, important pharmaceutical equipment and consumables, and precision scientific research instruments. and material R&D innovation. R&D and manufacturing of innovative drugs, focusing on innovative breakthroughs and large-scale production, develop immune cell therapy, proteins and peptides, antibody conjugates and other biotechnology drugs and new vaccines, and accelerate immunotherapy, gene therapy, oncolytic virus therapy and other technical products research and translation.
V. On April 20, 2021, the General Office of the Shanghai Municipal People's Government issued several opinions on promoting the high-quality development of the biomedical industry in this city. Support fields include pharmaceutical fields, which mainly include high-end biological products such as antibody drugs, new vaccines, gene therapy, and cell therapy, innovative chemical drugs and high-end preparations, and modern Chinese medicine.
Jiangsu Province
1. At the beginning of September 2021, the General Office of the Jiangsu Provincial Government issued the "Jiangsu Province's 14th Five-Year Plan for Scientific and Technological Innovation".
For the biomedical industry, it is pointed out that biomedical technology is a new engine for a new round of scientific and technological revolution and industrial transformation after information technology. Seize the strategic opportunity of the basic maturity of the global biological underlying technology and the accelerated breakthrough of major applications. Focus on the characteristics of "leading, groundbreaking, and disruptive", focus on the development of new generation gene editing, new sequencing, immune regulation, new biomedical imaging, new antibodies and vaccines and other cutting-edge technologies, and accelerate breakthroughs in chemical drugs, biotech drugs, and modern Chinese medicine. , special medical food and other key technologies, research and develop innovative drugs and high-end medical devices with independent intellectual property rights. Accelerate to build our province into a source of innovation in the biomedical industry with global influence. Cultivate and strengthen biotech drugs, focusing on the development of therapeutic antibodies, new vaccines, nucleic acid drugs, recombinant protein peptide drugs, genetic engineering drugs, cell therapy products, bacterial drugs and oncolytic viruses, overcome upstream and downstream technical bottlenecks, and strive to achieve high-end cell culture media , bioreactors, key core enzyme preparations, protein purification fillers, engineered cell lines, nanofiltration membranes, etc., to accelerate the industrialization process and quickly form a scale, becoming a new engine for the innovation and development of the biopharmaceutical industry in our province.
2. In August 2021, the General Office of the Jiangsu Provincial Government issued a notice on the "14th Five-Year Plan for High-Quality Development of Manufacturing Industry in Jiangsu Province".
Take the biomedical cluster as one of the development priorities during the "14th Five-Year Plan" period, and focus on improving the level of innovation and R&D as far as biological drugs are concerned. For malignant tumors, diseases of the immune system, etc., promote the research and development of new target biomacromolecule drugs such as therapeutic antibodies and cellular immunotherapy, accelerate the research and development of recombinant insulin, recombinant coagulation factors, recombinant granulocyte colony-stimulating factors and enzymes to replace recombinant protein drugs, actively Develop therapeutic vaccines, new coronavirus vaccines, influenza vaccines, AIDS vaccines and other major disease vaccines, encourage the development and industrialization of gene therapy drugs, and support the construction of a national biopharmaceutical technology innovation center. Taizhou is a pilot project for the cluster development of national-level new vaccines and specific diagnostic reagents.
Tianjin
1. On November 12, 2021, the "14th Five-Year Plan for the Development of Tianjin Biopharmaceutical Industry" was issued.
The article points out that it is necessary to build a biopharmaceutical industry cluster, promote the popularization and large-scale development of gene technology applications, and prospectively deploy biological products such as antibody drugs, antibody conjugated drugs, new structural protein and peptide drugs, nucleic acid drugs, and system target drugs. Promote tumor vaccines, AIDS vaccines, new crown vaccines and other new vaccines, as well as the original innovation of drugs for the treatment of cardiovascular and cerebrovascular diseases, neurodegenerative diseases, diabetes, autoimmune diseases, blood system and other major diseases; develop stem cell organ regeneration drugs , bispecific antibody drugs, antibody conjugated drugs and other new types of monoclonal antibody drugs. Support the introduction and production of high-end generic drugs and first-time generic drugs, and improve the quality of generic drugs for genetic and regenerative medicine. Implement industrial chain integration development promotion projects, promote the construction of application and transformation platforms for key biomedical products, cultivate specialized service platforms such as gene therapy, cell therapy, and immunotherapy that conform to international standards, and accelerate the application and transformation of new treatment technologies. Support high-end gene synthesis, gene editing and other professional technical service institutions, and promote professional services for new generic technologies. Accelerate the creation of new biopharmaceutical brands of "Weiyao", promote the creation of product brands in emerging fields such as genetic drugs, cell drugs, high-end medical devices, and smart medical care, vigorously promote leading enterprises and outstanding entrepreneurs, and stimulate the enthusiasm of corporate brand cultivation. Support outstanding enterprises in the field of biomedicine to participate in international competition, and support the city's leading traditional medicine enterprises, modern Chinese medicine enterprises, and emerging enterprises in the field of cells and genes to integrate research and development, market, production and other resources on a global scale. Overseas listing, etc., to open up the international market, realize the global layout of the industrial chain, and comprehensively enhance the international competitiveness of the enterprise. Support biopharmaceutical enterprises with high development potential to "go global" and carry out foreign investment and cooperation.
2. At the beginning of November 2021, the Tianjin Pilot Free Trade Zone Management Committee issued the "Reply on Approving the Construction of the China (Tianjin) Pilot Free Trade Zone Linkage Innovation Demonstration Base in the National Stem Cell Engineering Product Industrialization Base".
Build China (Tianjin) Pilot Free Trade Zone Linkage Innovation Demonstration Base in the National Stem Cell Engineering Product Industrialization Base. Biotechnology (Tianjin) Co., Ltd. and Tianjin Hechuang Biotechnology Co., Ltd. are the main bodies to explore and carry out gene and cell therapy pilot projects. The pilot period is 3 years from the date of approval. China (Tianjin) Pilot Free Trade Zone Linkage Innovation Demonstration Base will rely on the fully closed-loop management environment for cell storage, R&D, production and preparation, and clinical transformation and application of the National Stem Cell Engineering Product Industrialization Base, benchmarking against international common practices and standards, and in effective prevention and control Under the conditions of risk, organize and carry out the clinical transformation and application pilot of cell therapy, for the national trial system and trial standard, to create welfare and proliferation opportunities for patients, and to seek development and expand space for the industry. On the basis of full demonstration and risk prevention and control, boldly venture, boldly test, and make independent reforms. Promote the formation of a number of technical specifications or standards for cell collection, production and preparation, transportation, quality control, in-hospital release, risk control, etc. Innovative achievements; explore breakthroughs in the concurrent use of cell therapy clinical trials, the import of foreign and domestic unmarketed drugs and medical devices, and the application of cell therapy for clinical charges in accordance with medical technology access; form a batch of replicable and popularized pilot experiences , to lay a solid foundation for further expansion of the pilot scope.
Guangzhou City
1. On December 3, 2021, the Guangzhou Yuexiu District People's Government Office officially issued the "Notice on the 14th Five-Year Plan for the Development of Science and Technology Innovation and Strategic Emerging Industries in Guangzhou Yuexiu District".
The plan clearly states: precision medicine. Focus on the development of precision medicine in the fields of tumors, urinary system, cardiovascular system, digestive system, blood, and eye diseases, and vigorously support the application of new technologies such as stem cell therapy, tumor immunotherapy, and gene therapy. Actively guide domestic and foreign leading enterprises in the fields of gene technology, biotechnology and other fields to carry out strategic cooperation with large tertiary hospitals in the region, and build a precision treatment center in South China with comprehensive precision medical services. Introduce advanced technology, high-end equipment, leading scientific research talents and technical teams at home and abroad, promote the construction of an integrated industry-university-research cooperation platform and a genetic technology service center, build a new health care support system, and promote precision medicine in scientific research, achievement transformation, and clinical application. , personnel training and gene technology promotion and application development, form a gene technology application demonstration network with wide coverage and strong service capabilities, and build a precision medical and genetic testing base that serves Guangzhou and radiates the Greater Bay Area. Support the research and development of key core technologies. Focusing on key industrial fields such as new-generation information technology, artificial intelligence, digital economy, biomedicine, and modern service industries, as well as in areas such as urban governance and people's livelihood technology, strengthen key core technology research, and strive to enter the national layout in several key technology fields. Focus on life sciences, ultra-high-definition video and other fields to organize the implementation of a number of key R&D projects, accelerate the integrated development of key technologies such as modern genetic engineering drugs, antibody drugs, and new vaccines and new product development, and make breakthroughs in a number of key core technologies that restrict the development of key fields. And "stuck neck" technical problems, promote the rapid development of strategic emerging industries. Closely follow the major scientific and technological needs of the development of strategic emerging industries, focus on breaking through a number of key core technologies, research and develop a number of major strategic products, and cultivate and expand a number of innovative industrial clusters and leading backbone enterprises.

Shenzhen
1. On November 12, 2021, Shenzhen's important legislation in emerging areas, "Shenzhen Special Economic Zone Cell and Gene Industry Promotion Regulations (Draft for Comment)", was publicly solicited on the website of the Shenzhen Municipal People's Congress Standing Committee.
It regulates the collection and storage of cells, specifies the system for extended clinical trials of cells and genetic drugs, and encourages the development and use of genetic technology. The "Explanation on the "Shenzhen Special Economic Zone Cell and Gene Industry Promotion Regulations (Draft for Comment)" document pointed out that the cell and gene industry is the main development direction of the biomedical industry in the future, but Shenzhen's cell and gene industry is closely related to some domestic provinces and cities. Compared with the relatively slow development, there are still some bottlenecks that restrict the development and growth of the industry, such as basic research to be strengthened, the industrial chain is not perfect, medical institutions cannot meet the needs of clinical research, and the approval efficiency needs to be improved. It is proposed that the municipal and district people's governments should give full play to the supporting role of government investment funds in industrial development, guide social funds to be invested in the cell and gene industry, and promote the progress of key links such as product research and development and transformation of achievements. Encourage financial institutions to provide financial support for the development of cell and gene industries, increase credit support, and reduce corporate financing costs. Support eligible cell and gene companies to go public. Explore the provision of intellectual property financial services such as intellectual property pledge financing and intellectual property insurance for cell and gene companies, and develop intellectual property securitization financing products.
Zhejiang Province
1. According to the news on the website of the Zhejiang Provincial Health Commission on November 23, the Zhejiang Provincial Health Commission recently replied to Dai Lingzhi, a representative of the Provincial People's Congress, "On the first trial of cell technology research and application in China (Zhejiang) Pilot Free Trade Zone. Suggest"
. According to the document, based on the relevant circumstances and the recent approval of CAR-T cell therapy products by the State Food and Drug Administration, cell therapy no longer has the problem of clinical transformation, but should be used as a drug and enter clinical application after being approved by the drug regulatory department. The Zhejiang Provincial Health and Health Commission has provided policy support to accelerate the creation and industrialization of new drugs in the field of cell therapy based on clinical needs. In the next step, the Zhejiang Provincial Health Commission and relevant departments will implement the "Notice of the State Council on Printing and Distributing the Overall Plan for China (Zhejiang) Pilot Free Trade Zone" (Guo Fa [2017] No. 16) and the "Zhejiang Provincial People's Government on Printing and Distributing China (Zhejiang) Pilot Free Trade Zone." Zhejiang) Circular on the Implementation Plan for Deepening Reform and Opening-up in the Pilot Free Trade Zone (Zhezheng Fa [2020] No. 32), actively supporting cell therapy-related technology research, strengthening policy tracking research, and standardizing access management.
Hangzhou
On February 19, 2021, the People's Government of Xiaoshan District, Hangzhou City officially released the "Construction Plan for Xiaoshan Block in Hangzhou Area of China (Zhejiang) Pilot Free Trade Zone" on its official website.
It is clearly stated in the plan: to explore biomedicine first and foremost. Promoting pilot projects in biopharmaceutical review and approval, import and export customs clearance, medical services, data and talent element guarantees. Focus on opening up the approval authority for clinical and commercialization of cell therapy and gene therapy, and explore the establishment of a green channel in the process of application and testing of cell and gene therapy drugs.
Dalian
On April 19, 2021, the Dalian Free Trade Zone signed a framework agreement with Dalian Medical University to jointly build the "Dalian Gene Cell Therapy Pilot Demonstration Zone" in the Dalian Free Trade Zone.
According to the agreement, the two parties will build a clinical medical center for cell therapy, and promote the first trial of cell therapy in the Dalian Free Trade Zone. Give full play to the advantages of Dalian Medical University's disciplines, high-level talents and high-level medical treatment, focusing on in-depth research in the fields of cell therapy, genetic testing, and drug research and development. Explore the establishment of a research and development and achievement transformation model that adapts to the characteristics of the Dalian Free Trade Area, strive to realize the transformation and application of key core technologies in the area, and actively carry out external cooperation in gene and cell therapy.
Kunming
On March 11, 2021, the Cell Industry Policy Conference of Kunming National High-tech Industrial Development Zone (hereinafter referred to as "Kunming High-tech Zone") and the listing ceremony of the Kunming High-tech Zone Cell Industry Alliance were held. Several Policies for Cluster Innovation and Development (for Trial Implementation).
The policy clearly states that 16 policies and measures have been introduced around seven aspects, including supporting the development of cell industry clusters, promoting the standardization of cell industry, and supporting the construction of cell industry platforms. cluster area, and strive to promote the development of cell industry clusters.
City of Yantai
On August 20, 2021, Yantai City issued the "Several Opinions of the Yantai Municipal People's Government on Promoting the High-Quality Development of the Biomedical Industry in the City".
Indicate in the general requirements section: development goals. Focus on eight sub-sectors: innovative drugs, high-end APIs, rehabilitation aids and high-end medical devices, traditional Chinese medicine, special medical food and health food, medical and health big data, medical beauty products, and cell and gene therapy. Focus on building key technology service platforms such as generic technology research platforms, pharmaceutical big data service platforms, clinical trial service platforms, R&D and production service platforms; promote the establishment of a cell and gene therapy development alliance, and build a "China-Japan Regenerative Medicine Clinical Trial Center" and "Stem Cells" Stem cell and regenerative medicine research and development platforms and achievement transformation platforms such as Regenerative Medicine and Anti-aging Research Center. Key tasks: In the field of innovative drugs, focus on major diseases such as malignant tumors, cardiovascular and cerebrovascular diseases, metabolic diseases and neuropsychiatric diseases, and develop a series of high-end innovative products such as new antibody drugs, innovative preparations and new drug delivery systems. Research, development and production; promote the development of cutting-edge technologies such as gene and cell therapy and new vaccines. In Yantai's several policies to promote the high-quality development of the biopharmaceutical industry, it is pointed out that in the field of drugs, focus on supporting high-end biological drugs such as antibody drugs, antibody-conjugated drugs, new vaccines, gene therapy, and cell therapy, innovative chemical drugs and high-end preparations, and traditional Chinese medicine. Innovative medicines and traditional Chinese medicine compound preparations with ancient classics and famous prescriptions, etc. Encourage social capital to participate in the construction of public service platforms. Contract research and development institutions (CROs) in the fields of newly established and certified innovative drugs, specialty APIs, rehabilitation aids and high-end medical devices, traditional Chinese medicine, special medical food and health food, medical beauty products, cell and gene therapy, etc. Professional technology platforms such as contract outsourcing production organizations (CMOs), contract custom R&D and production organizations (CDMOs), or new equipment on existing platforms will be subsidized by 20% of the total purchase of platform equipment.

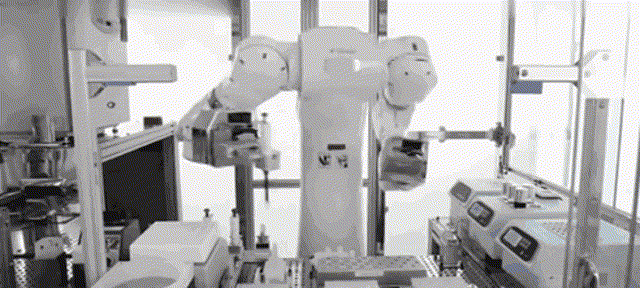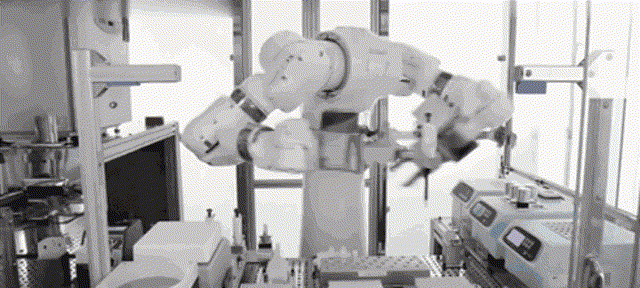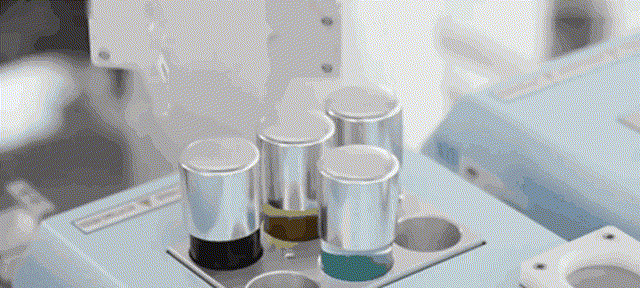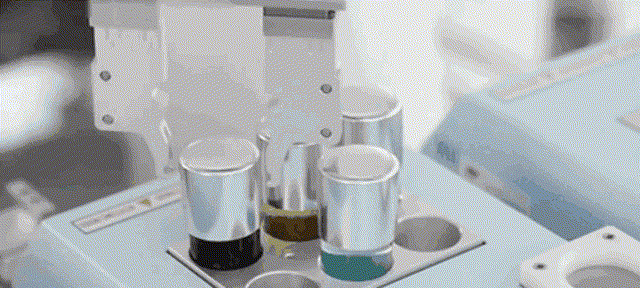
Although most of the medical scientific research equipment for the production and preparation of stem cells has been monopolized by foreign technology for a long time and has to rely on imports, Robot 365 adheres to the R&D and design concept of allowing artificial intelligence technology to create medical value, and has breakthrough application scenarios in this field. Revolutionary historical significance, assisting the development of the stem cell industry and making great contributions! Under the vigorous promotion of national policies, the industries of stem cell therapy, immune cell therapy, gene therapy and gene editing are about to develop rapidly. I believe that in the near future, China's stem cell industry will reach a world-class level!